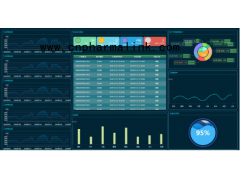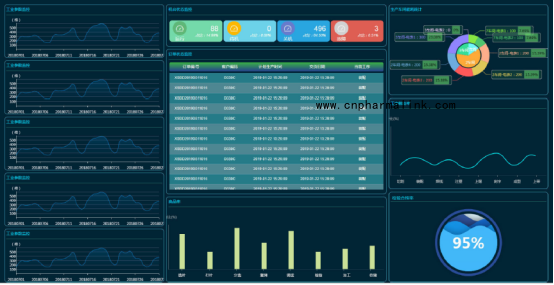
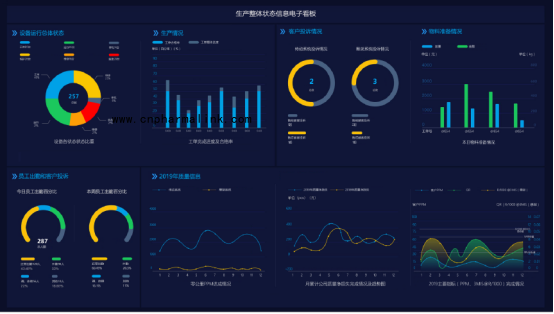
Equipment overview
Based on the pharmaceutical industry, honray MES is committed to realizing digital informatization of pharmaceutical enterprises. Complete product functions, with good versatility, flexibility and scalability, suitable for the production management of preparations, raw materials, Chinese patent medicines, biopharmaceuticals and other drugs.
According to the real-time production status, the production is finely scheduled, and the personnel, equipment, materials, environment, and procedures of each link of the pharmaceutical production are organically combined to realize the standardization, electronic and visualization of the entire production process, ensuring the reliability and integrity of the production data, and effectively reducing quality risks, improve efficiency, and enhance the competitiveness of pharmaceutical companies.
Product module
SCADA data acquisition control system: including equipment acquisition and status display module, video remote monitoring module, alarm and alarm processing module, accident trace and trend analysis module, environmental data acquisition module, EMS energy management module, OEE analysis, access control acquisition module, production Process technology parameter acquisition module, production process control module and other modules.
MES Manufacturing Execution System: includes basic data authority management module, personnel team management module, electronic batch record and verification module, equipment management module, recipe management module, electronic weighing ingredient management module, inventory material circulation management module, production scheduling module , Quality deviation management module, audit release management module, production execution module, quality traceability module, GMP document management module, SCADA integration module, statistical report analysis module.
Other optional system modules:
●LIMS Laboratory Information Management System
●Intelligent solutions for stereoscopic warehouses
● Automation transformation services for docking module equipment of
ERP, financial, SCM and other peripheral system
●Other information system customization services
Product features
Seamless integration of SCADA data acquisition and monitoring, designed for the pharmaceutical industry 4.0.
Based on cGMP regulations, with electronic batch records as the core, significantly improve the efficiency of each stage of pharmaceutical production.
Fully service-oriented architecture, high scalability, to meet the information needs of different development stages in the enterprise development process.
Multi-terminal adaption, desktop end, WEB end, PAD end, mobile end, WeChat end, each end highly adaptable, all for better user experience.
Provide multi-dimensional and rich data analysis, with highly customized push service, all for better information mining and more effective information transmission.
Provide ERP, SCM, OA pharmaceutical enterprises with existing system integration services.
Independent research and development products can fully meet the customized needs of users.